Products / Application / Protein Analysis
Products
Cell-free Protein Expression
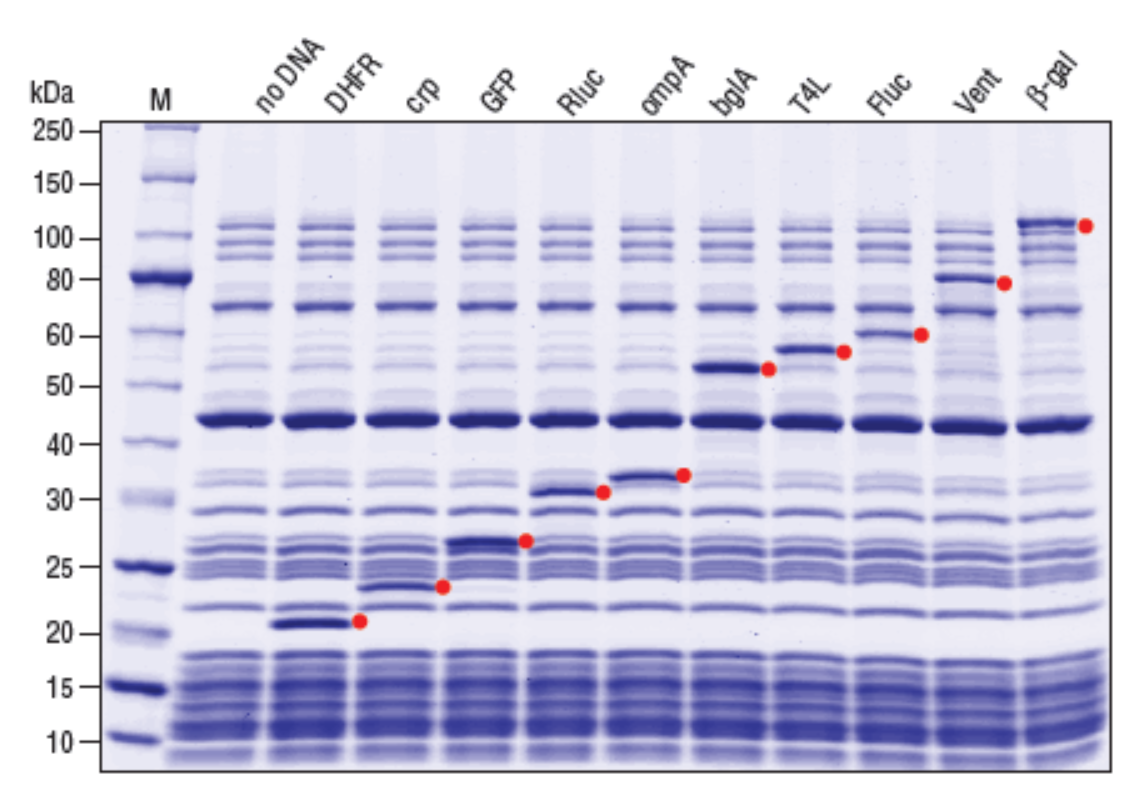
A reconstituted protein synthesis system based on the PUREsystem™ (Shimizu et al., 2001) where all necessary components needed for in vitro transcription and translation are purified from E. coli.
- Defined system with all his-tagged proteins for coupled transcription/translation; Ribosome is not his-tagged
- T7 RNA Polymerase drives in vitro transcription
- Minimal nuclease and protease activity for stability of synthesized protein and encoding target
- Templates can be either plasmid DNA, linear DNA or mRNA
- Protein of interest can be synthesized and visualized in a few hours
- Synthesized protein can be co-translationally radiolabeled or fluorescently labeled
- Protein can be reverse-purified or subject to direct functional analysis
- Applications include high throughput screening/directed evolution, synthetic biology, toxic or difficult to express protein synthesis, studies on protein folding, activity and protein-protein interactions
- Due to reconstituted nature, several kits are offered where translation factors or macromolecules have been omitted to facilitate specific studies (see companion products)
- Compatible with the PURExpress Disulfide Bond Enhancer (NEB #E6820)
More Information:
Competent Cells for Protein Expression

Chemically competent E. coli cells suitable for tunable T7 expression of challenging proteins.
- BL21(DE3) containing the Lemo System™
- Tunable T7 Expression Strain for difficult targets: membrane proteins, toxic proteins and proteins prone to insoluble expression
- Deficient in proteases Lon and OmpT
- Resistant to phage T1 (fhuA2)
- Free of animal products
More Information:
Protein Purification

An affinity matrix for the small-scale isolation and purification of polyhistidine-tagged (His-tagged) fusion proteins in manual or automated formats.
- Isolation and purification of His-tagged fusion proteins under native or denaturing conditions as IMAC tolerates a wide range of conditions, including the presence of protein denaturants and detergents.
- High specific binding of His-tagged proteins from various expression systems with purities of >95%.
- NTA securely coordinates metal ions through four coordination sites which results in low nickel ion leaching.
- Can be used with common cell lysis reagents and a variety of buffer additives.
More Information:
Protein Standards
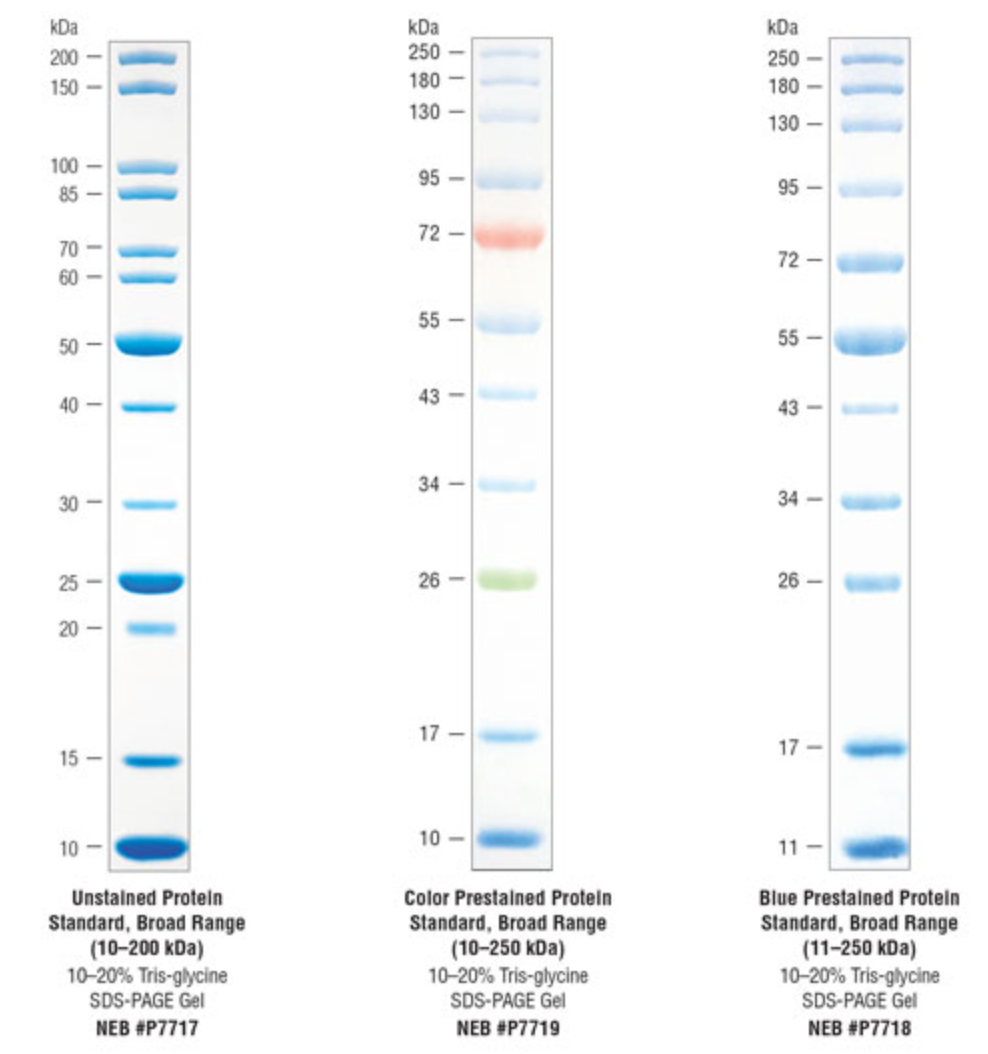
- Direct loading, additional loading buffer and heat incubation not required
- Applications include verification of western blot transfer efficiency on membranes and fluorescent imaging of SDS-PAGE
More Information:
- Markers & Ladders Products – selection of DNA, RNA and protein markers and ladders
Glycoproteomics
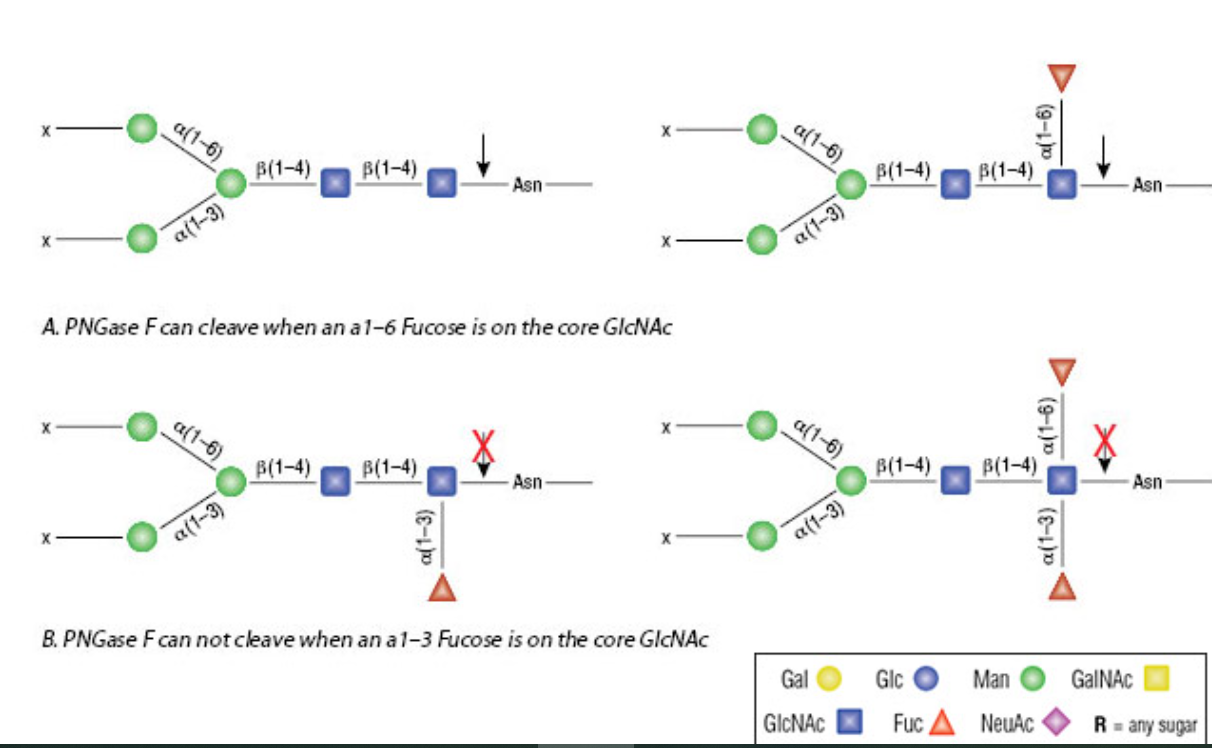
Removal of high mannose N-glycans from glycoproteins
- Non-recombinant with no detectable endoglycosidase F1, F2 or F3 contamination
- ≥ 95% purity, as determined by SDS-PAGE and intact ESI-MS
- Stored in 50% glycerol
- Optimal activity and stability for up to 24 months
- Can be used under native or denaturing conditions
More Information:
